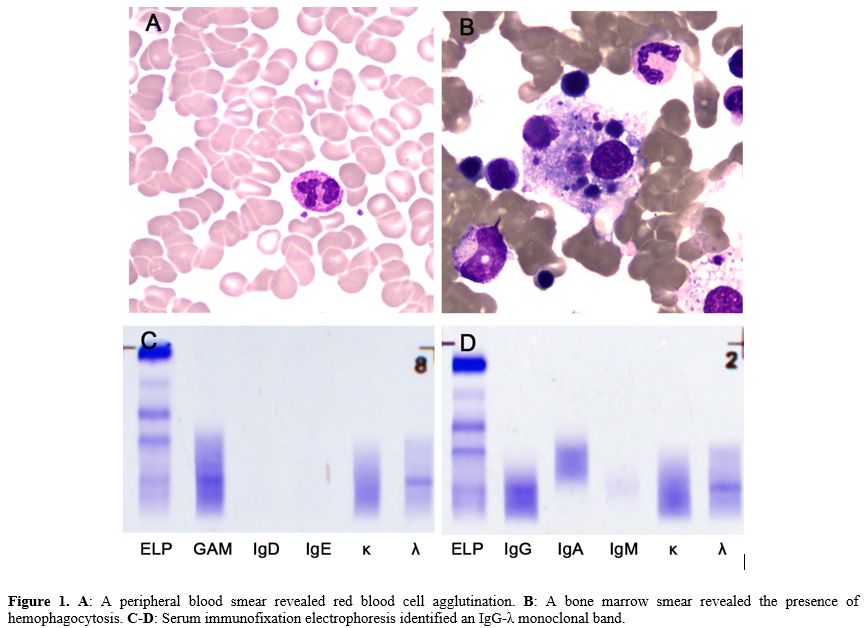
A 78-year-old male with a history of brain infarction was admitted with a 3-day history of fever and progressively worsening dyspnea. Laboratory tests revealed hemoglobin levels of 8.7 g/dL (reference, 13.0–17.5 g/dL), red blood cell (RBC) count of 1.75×1012/L (reference, 4.3–5.8×1012/L), reticulocyte percentage of 4.32% (reference, 0.5%–1.5%), white blood cell count of 10.37×109/L (reference, 3.5–9.5×109/L), and platelets of 227×109/L (reference, 85–303×109/L). Liver function tests showed elevated total bilirubin of 46.6 μmol/L (reference, 0–23 μmol/L), indirect bilirubin of 32.4 μmol/L (reference, 5–18 μmol/L), and lactate dehydrogenase of 413 U/L (reference, 120–250 U/L). A direct antiglobulin test and cold-reactive autoantibody were positive. This antibody did not react at physiological temperature but reacted with patient and donor RBCs at cold temperatures. Peripheral smear revealed RBC agglutination (Figure 1A). A saliva-based reverse-transcription polymerase chain reaction test confirmed the presence of SARS-CoV-2 infection with a viral load of 624 copies. Additional COVID-19-related markers, including ferritin (1928 ng/mL), D-dimer (9.83 mg/L), C-reactive protein (127.4 mg/L), and interleukin-6 (167.78 pg/mL), were all elevated, highlighting the systemic inflammatory response associated with COVID-19 infection.
Further investigation into the patient's anemia revealed a bone marrow smear showing hemophagocytosis, a phenomenon wherein activated macrophages engulf blood cells, a feature often seen in severe inflammatory or infectious conditions (Figure 1B). There was no plasma cell or lymphoplasmacytic cell infiltration, ruling out multiple myeloma or other plasma cell disorders. Serum immunofixation electrophoresis identified an IgG-λ monoclonal band, indicative of an underlying monoclonal gammopathy (Figures 1C-D). Importantly, the workup for other common causes of hemolytic anemia, including tests for antinuclear antibody, rheumatoid factor, Mycoplasma pneumoniae, Epstein–Barr virus, parvovirus, human immunodeficiency virus, and acute hepatitis, was negative. Furthermore, abdominal and pelvic computed tomography scans did not reveal any signs of lymphadenopathy or solid tumors. As a result, glucocorticoids were administered. Unfortunately, his course was complicated by hypoxic respiratory failure and refractory septic shock, and the patient died after his family was elected for comfort-oriented care.
Cold agglutinins are autoantibodies that target RBC antigens, causing hemolysis at temperatures lower than normal body temperature through complement fixation. Their production can be triggered by infections, leading to secondary CAS. This syndrome is commonly associated with Mycoplasma pneumoniae infections, as well as various viral infections.[5] In COVID-19 patients, the mechanism of CAS may be related to the formation of autoantibodies induced by viral infection, which bind to red blood cells at low temperatures, resulting in hemolysis.[6] In this case, the patient presented with not only CAS but also hemophagocytosis, and MGUS, which could be pre-existing.
Several theories have been proposed regarding the link between autoimmune diseases and COVID-19. One hypothesis is that SARS-CoV-2 may induce autoimmunity by mimicking self-antigens, thereby disrupting immune tolerance.[7] Additionally, COVID-19 may amplify the production of pre-existing cold agglutinins and autoantibodies against red blood cells.[8] Viruses, in general, are closely linked to the development of hemophagocytic lymphohistiocytosis, often acting as infectious triggers. A key mechanism in the pathogenesis of immune-mediated conditions triggered by viral infections is the loss of tolerance to self-antigens. Studies in COVID-19 patients suggest that monocytes and macrophages play a critical role in the progression of the virus.[9] MGUS is associated with an immune dysfunction similar to that of multiple myeloma, including significant defects in both humoral and cellular immunity.[10] As a result, individuals with MGUS are at an increased risk for infections, including viral infections.[11] While MGUS has been linked to increased susceptibility to infections, its impact on COVID-19 severity remains inconclusive. Although some have speculated that MGUS might influence the risk of SARS-CoV-2 infection and the severity of COVID-19,[12] large population-based cohort studies and case series have not found a significant association between MGUS and either susceptibility to SARS-CoV-2 or the severity of COVID-19.[13,14]
Diagnosing CAS and AIHA COVID-19-related requires a comprehensive approach.[15] In this case, the patient exhibited significant erythrocyte agglutination and bone marrow hemophagocytosis, indicating severe immune-mediated hemolysis and inflammation. Additionally, inflammatory markers commonly elevated in COVID-19, such as ferritin, D-dimer, and interleukin-6, were markedly increased, further supporting the diagnosis of immune activation.[9] Clinical management of such complex cases involves a multi-faceted approach, addressing both the COVID-19 infection and autoimmune hemolysis while monitoring for potential complications related to MGUS. Both hemolytic anemia with CAS or CAD may benefit from complement inhibitors that are also under study for COVID-19 disease.[3] Treatment wth prednison is actve in CAD related AIHA, for CAS specifically, targeted therapies such as rituximab or the complement inhibitor pegcetacoplan may be required.[16]
In conclusion, we report a rare case of COVID-19-associated cold agglutinin syndrome and hemophagocytosis in a patient with MGUS. This case emphasizes the importance of considering autoimmune and hematologic complications in COVID-19 patients, particularly those with pre-existing immune dysfunction.
Ethics Approval
The study protocol was approved by the Medical Ethics Committee of the Zigong First People’s Hospital.Informed Consent
Written informed consent was obtained from the patient's legal guardians.References
- Lazarian G, Quinquenel A, Bellal M, Siavellis J,
Jacquy C, Re D, Merabet F, Mekinian A, Braun T, Damaj G, Delmer A,
Cymbalista F. Autoimmune haemolytic anaemia associated with COVID-19
infection. Br J Haematol. 2020;190:29-31. https://doi.org/10.1111/bjh.16794 PMid:32374906 PMCid:PMC7267601
- Hindilerden
F, Yonal-Hindilerden I, Akar E, Yesilbag Z, Kart-Yasar K. Severe
Autoimmune Hemolytic Anemia in COVID-19 Infection, Safely Treated with
Steroids. Mediterr J Hematol Infect Dis. 2020;12:e2020053. https://doi.org/10.4084/mjhid.2020.053 PMid:32670531 PMCid:PMC7340241
- Fattizzo
B, Pasquale R, Bellani V, Barcellini W, Kulasekararaj AG. Complement
Mediated Hemolytic Anemias in the COVID-19 Era: Case Series and Review
of the Literature. Front Immunol. 2021;12:791429. https://doi.org/10.3389/fimmu.2021.791429 PMid:34899761 PMCid:PMC8655106
- Huscenot
T, Galland J, Ouvrat M, Rossignol M, Mouly S, Sene D, Group ALC.
SARS-CoV-2-associated cold agglutinin disease: a report of two cases.
Ann Hematol. 2020;99:1943-4. https://doi.org/10.1007/s00277-020-04129-9 PMid:32591877 PMCid:PMC7317069
- Jager
U, Barcellini W, Broome CM, Gertz MA, Hill A, Hill QA, Jilma B, Kuter
DJ, Michel M, Montillo M, Roth A, Zeerleder SS, Berentsen S. Diagnosis
and treatment of autoimmune hemolytic anemia in adults: Recommendations
from the First International Consensus Meeting. Blood Rev.
2020;41:100648. https://doi.org/10.1016/j.blre.2019.100648 PMid:31839434
- Maslov
DV, Simenson V, Jain S, Badari A. COVID-19 and Cold Agglutinin
Hemolytic Anemia. TH Open. 2020;4:e175-e7.
https://doi.org/10.1055/s-0040-1715791 PMid:32844144 PMCid:PMC7440967
- Yazdanpanah N, Rezaei N. Autoimmune complications of COVID-19. J Med Virol. 2022;94:54-62. https://doi.org/10.1002/jmv.27292 PMid:34427929 PMCid:PMC8661629
- Tsukamoto
Y, Umeda M, Muto Y, Sugimoto T, Yamauchi M, Ando K, Ariyoshi K. Severe
Anemia Due to Cold Agglutinin Syndrome in a COVID-19 Patient with IgM
Monoclonal Gammopathy of Undetermined Significance Successfully Treated
with Corticosteroids. Intern Med. 2022;61:1789-93. https://doi.org/10.2169/internalmedicine.8647-21 PMid:35342131 PMCid:PMC9259319
- Retamozo
S, Brito-Zeron P, Siso-Almirall A, Flores-Chavez A, Soto-Cardenas MJ,
Ramos-Casals M. Haemophagocytic syndrome and COVID-19. Clin Rheumatol.
2021;40:1233-44. https://doi.org/10.1007/s10067-020-05569-4 PMid:33389315 PMCid:PMC7778844
- Tete
SM, Bijl M, Sahota SS, Bos NA. Immune defects in the risk of infection
and response to vaccination in monoclonal gammopathy of undetermined
significance and multiple myeloma. Front Immunol. 2014;5:257. https://doi.org/10.3389/fimmu.2014.00257 PMid:24917865 PMCid:PMC4042361
- Kristinsson
SY, Tang M, Pfeiffer RM, Bjorkholm M, Goldin LR, Blimark C, Mellqvist
UH, Wahlin A, Turesson I, Landgren O. Monoclonal gammopathy of
undetermined significance and risk of infections: a population-based
study. Haematologica. 2012;97:854-8. https://doi.org/10.3324/haematol.2011.054015 PMid:22180421 PMCid:PMC3366650
- Jain
A, Ramasamy K. Potential 'significance' of monoclonal gammopathy of
'undetermined significance' during COVID-19 pandemic. Blood Cells Mol
Dis. 2020;85:102481. https://doi.org/10.1016/j.bcmd.2020.102481 PMid:32745940 PMCid:PMC7377999
- Rognvaldsson
S, Eythorsson E, Thorsteinsdottir S, Vidarsson B, Onundarson PT,
Agnarsson BA, Sigurdardottir M, Thorsteinsdottir I, Olafsson I,
Runolfsdottir HL, Helgason D, Emilsdottir AR, Agustsson AS, Bjornsson
AH, Kristjansdottir G, Thordardottir AR, Indridason OS, Jonsson A,
Gislason GK, Olafsson A, Steingrimsdottir H, Kampanis P, Hultcrantz M,
Durie BGM, Harding S, Landgren O, Palsson R, Love TJ, Kristinsson SY.
Monoclonal gammopathy of undetermined significance and COVID-19: a
population-based cohort study. Blood Cancer J. 2021;11:191. https://doi.org/10.1038/s41408-021-00580-7 PMid:34853309 PMCid:PMC8635472
- Gonzalez-Lugo
JD, Bachier-Rodriguez L, Goldfinger M, Shastri A, Sica RA, Gritsman K,
Mehta V, Kabarriti R, Goel S, Verma A, Braunschweig I, Kornblum N,
Mantzaris I. A case series of Monoclonal Gammopathy of Undetermined
Significance and COVID-19. Br J Haematol. 2020;190:e130-e3. https://doi.org/10.1111/bjh.16906 PMid:32479664 PMCid:PMC7300638
- Patil
NR, Herc ES, Girgis M. Cold Agglutinin Disease and Autoimmune Hemolytic
Anemia with Pulmonary Embolism as a Presentation of COVID-19 Infection.
Hematol Oncol Stem Cell Ther. 2022;15:213-6. https://doi.org/10.1016/j.hemonc.2020.06.005 PMid:32645300 PMCid:PMC7336954
- Roman
E, Fattizzo B, Shum M, Hanna W, Lentz SR, Araujo SSS, Al-Adhami M,
Grossi FV, Gertz MA. Safety and efficacy of pegcetacoplan treatment for
cold agglutinin disease and warm antibody autoimmune hemolytic anemia.
Blood. 2025;145:397-408. https://doi.org/10.1182/blood.2023022549 PMid:39486046